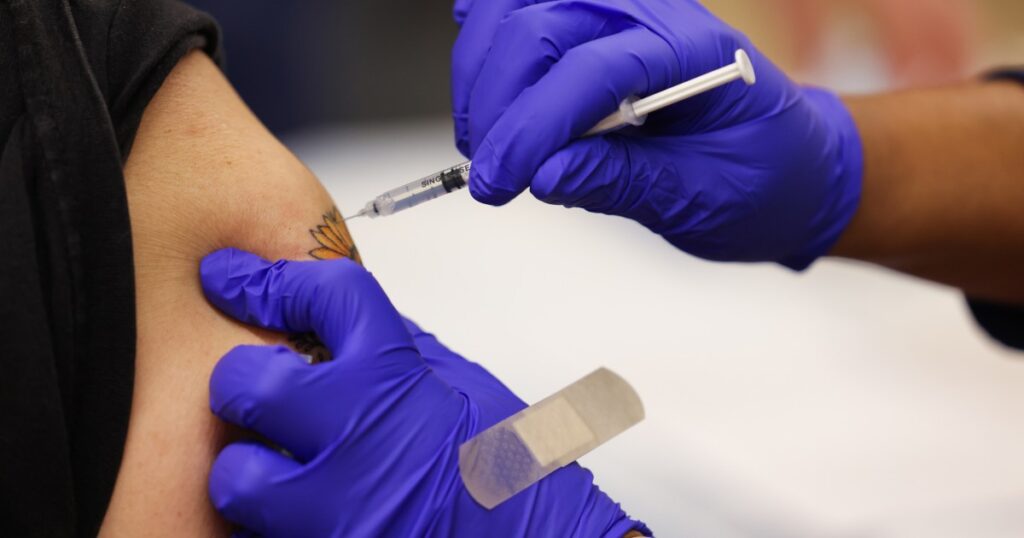
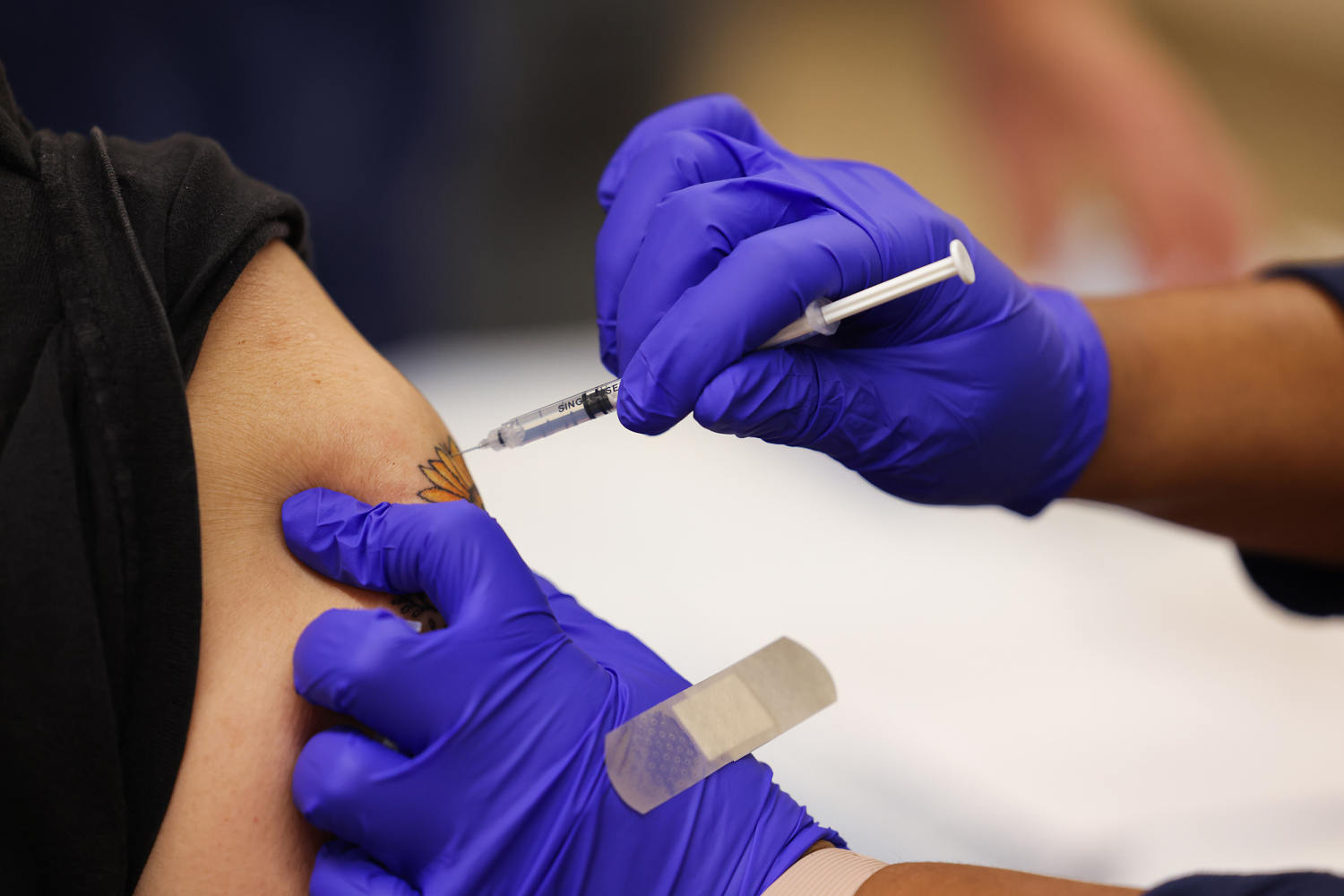
The anticipated rollout of updated Covid vaccines this fall might be at risk after a change by Health and Human Services Secretary Robert F. Kennedy Jr. in how vaccines are tested, experts say.
Under the change by Kennedy, according to an HHS spokesperson, all new vaccines will need to go through placebo-controlled clinical trials — where some people get the actual shot and others get something inactive, like a saline shot — to compare the results.
Running trials that include a placebo group is already routine for most new vaccines.
The original Covid vaccines, from Pfizer and Moderna, approved in late 2020, went through placebo-controlled trials. But as the virus continued to mutate and the vaccines needed to be updated to match the circulating strain, drugmakers moved to a flu vaccine-like model — using smaller studies to test how well the updated shots triggered an immune response against the variant in question.
Like the annual flu shot, the updated Covid vaccines weren’t treated as entirely new products, since they still used the same formula, with just a tweak to what strain the vaccine would be targeting. The mRNA Covid vaccines were designed so that this change would be particularly easy to make, in the event the shots needed to be quickly updated.
Quickly, in this case, turns out to be several months. In order to have enough Covid doses ready to go for the fall, vaccine-makers are told what strain to target in the spring.
The Food and Drug Administration’s vaccine advisory committee is expected to meet in May or June to make a recommendation on which strains should be included in the next round of shots. A person familiar with the matter, who was not authorized to speak publicly, said the FDA had planned to schedule a meeting for May 22. An HHS spokesperson declined to comment on the meeting date.
If the FDA deems Pfizer’s and Moderna’s updated vaccines “new” products, requiring fresh trials, it’s extremely unlikely the doses would be ready for the fall, experts said.
Dr. Paul Offit, the director of the Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA’s vaccine advisory committee, said the change would almost certainly delay the rollout of the updated shots from Pfizer and Moderna by “months,” as it would take time for the drugmakers to design the new trials and enroll participants.
That would only be the start — the drugmakers would then have to run the actual trial, which would take several months on its own, at minimum, and analyze the results.
Even the Covid vaccines — which were hailed as “the most successful government science program” because of how quickly they were developed, according to Dr. Alex Greninger, a professor of laboratory medicine and pathology at UW Medicine in Seattle — still took at least about six months to run their Phase 3 trials.
The HHS spokesperson didn’t directly respond to a question about whether the Pfizer and Moderna vaccines would require new clinical trials.
However, the spokesperson said in a statement that “FDA Commissioner Dr. Marty Makary has indicated that significant updates to existing vaccines — such as those addressing seasonal strain changes or antigenic drift — may be considered ‘new products’ requiring additional clinical evaluation.”
“As we’ve said before, trials from four years ago conducted in people without natural immunity no longer suffice,” the spokesperson said. “A four-year-old trial is also not a blank check for new vaccines each year without clinical trial data,” they said, adding that the flu shot would be exempt from the new rule, because it “has been tried and tested for more than 80 years.”
The FDA has already delayed the approval of Novavax’s updated Covid vaccine, requiring the company to carry out a new clinical trial because the strain included in the shot differs from what was originally authorized.
Vaccine experts panned the new requirements.
It’s “unethical,” Offit said, noting that it’s generally frowned upon in the scientific community to give someone a placebo when an approved product already exists that can protect them.
Dr. Stanley Plotkin, a pediatrician who played a key role in developing the rubella vaccine, said the move would make “no sense.”
“What would be reasonable is to compare the old vaccine with the new vaccine to see whether the new vaccine gives better immunologic responses,” Plotkin said. “We have vaccines against Covid, where we have pretty concrete ideas as to what works and what doesn’t work. We know they’re not perfect, but we have vaccines we know work.”
Spokespeople for Pfizer and Moderna did not immediately respond to requests for comment.
Former government officials have said HHS, under Kennedy, was moving to slow-walk vaccine approvals, including by imposing new regulatory hurdles on drugmakers, such as changing the requirements for approval or seeking additional clinical trial data.
Vaccine experts also fear the rule change is part of a broader effort by Kennedy to sow distrust in vaccines and limit public access to them.
“The goal is to make vaccines more onerous to make, more onerous to test by bringing up these sort of false safety concerns or false efficacy concerns,” Offit said.
Dr. Sean O’Leary, chair of the American Academy of Pediatrics Committee on Infectious Diseases, said the change is unlikely to affect brand-new vaccines. But it could have major implications for vaccines that may require updates besides Covid — like those for RSV — since placebo trials are costly and take significantly more time to conduct.
“It’s really not feasible, and would lead to lots of hospitalizations and deaths,” O’Leary said.
Plotkin added that vaccines being developed for infections that are incurable, like HIV, may also be at risk.
“Suppose you wanted to develop a new HIV vaccine?” he asked. “Would you do a placebo-controlled trial in that situation? I mean exposing children to a disease which is very serious without offering them anything.”
 Latest World Breaking News Online News Portal
Latest World Breaking News Online News Portal






