Trump, RFK Jr. launch $500M universal vaccine initiative
Fox News senior medical analyst Dr. Marc Siegel discusses the Trump administration’s initiative to develop a vaccine to target multiple viruses and the Murdoch Children’s Research Institute studying potential vaccines for strep.
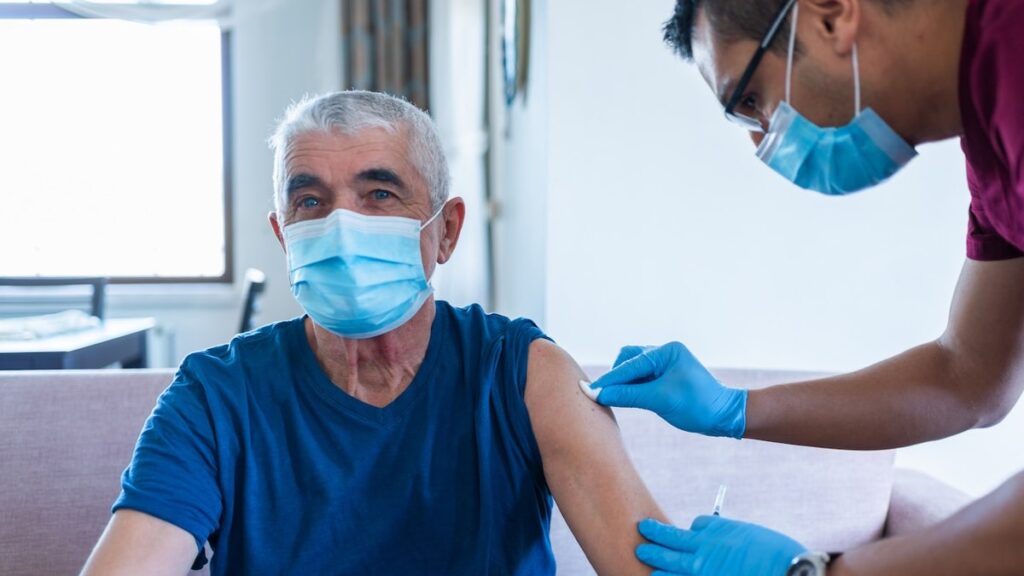
Older adults are being warned against receiving the chikungunya vaccine before traveling.
The Ixchiq vaccination, developed by Valneva to prevent the mosquito-borne chikungunya virus, was approved by the Food and Drug Administration (FDA) in November 2023 as the first of its kind.
The approval applies to anyone aged 18 and older who has a risk of being exposed to the virus.
FIRST VACCINE FOR CHIKUNGUNYA VIRUS, AN ‘EMERGING GLOBAL HEALTH THREAT,’ GETS FDA APPROVAL
But the FDA and the Centers for Disease Control and Prevention (CDC) released a safety notice on May 9 recommending that adults over 60 years old pause use of the vaccine due to fatal complications.
“FDA and CDC will continue the evaluation of post-marketing safety reports for Ixchiq,” the release reads.
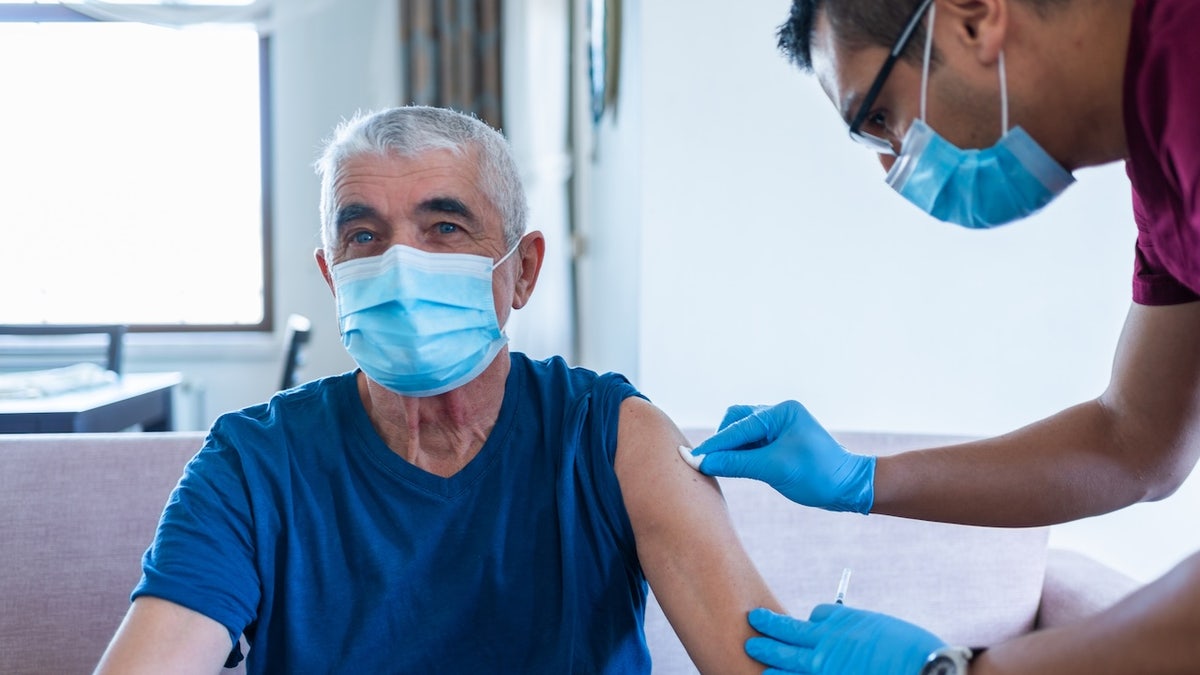
Older adults are being warned against receiving the chikungunya vaccine before traveling. (iStock)
“While the safety of Ixchiq for use in individuals 60 years of age and older is being further assessed, FDA and CDC are recommending a pause in use of the vaccine in this age group. FDA and CDC will update the public when the agencies complete their evaluation of this safety issue.”
The advisory follows reports of “serious adverse events,” including neurologic and cardiac events in people who received the vaccine.
CLICK HERE TO GET THE FOX NEWS APP
Two of 17 events resulted in death from severe complications. One death was caused by encephalitis, or inflammation in the brain, the alert stated.
Those who experienced adverse effects of the vaccine were reported to be between the ages of 62 and 89.
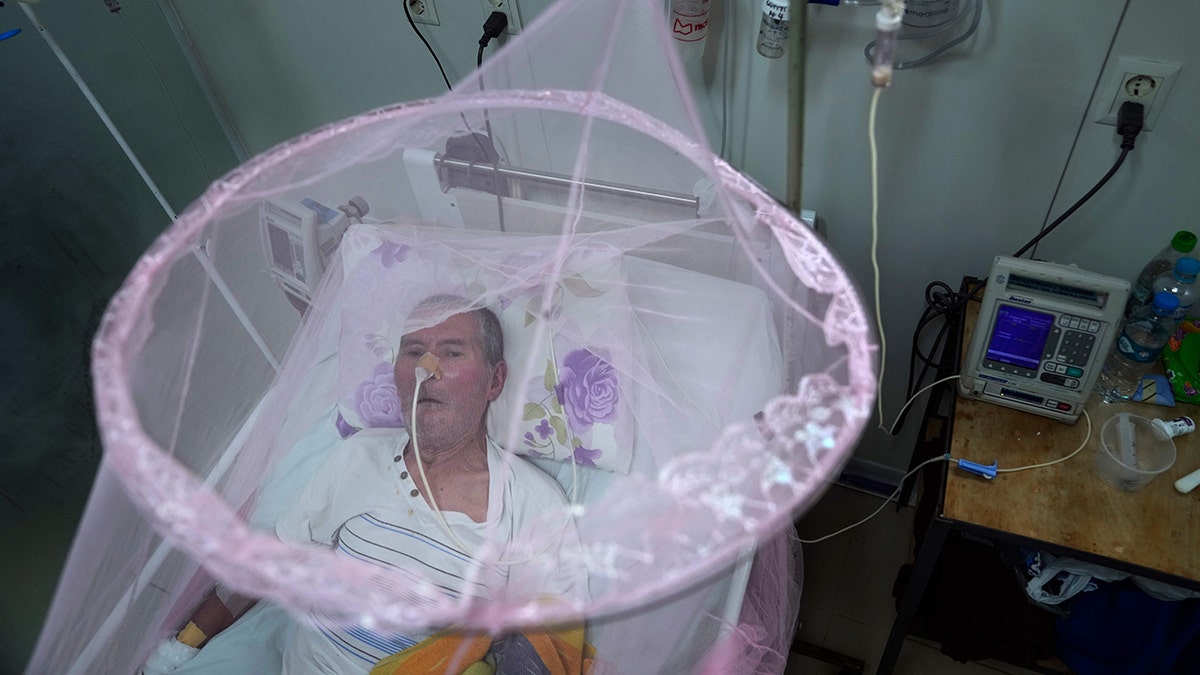
A patient infected with chikungunya looks out from mosquito netting at the Clinicas Hospital in San Lorenzo, Paraguay, in March 2023. The FDA warned that Ixchiq, which contains a live, weakened version of the virus, may cause similar symptoms to chikungunya. (AP Photo/Jorge Saenz)
The FDA warned that Ixchiq, which contains a live, weakened version of chikungunya, may cause symptoms similar to the virus.
Typical symptoms of chikungunya include fever, severe joint pain, headache, muscle pain and a rash, according to the CDC.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Most people recover within a week, but some may experience “severe and disabling” joint pain for weeks or months.

Chikungunya is spread by the bite of infected mosquitoes. (iStock)
“This virus is in a similar category as dengue or Zika and is carried by the same mosquitoes,” Fox News senior medical analyst Dr. Marc Siegel previously told Fox News Digital.
At the time of the vaccine’s approval, the FDA described chikungunya as an “emerging global health threat,” with at least five million cases reported over the past 15 years.
For more Health articles, visit www.foxnews.com/health
The FDA plans to conduct an “updated benefit-risk assessment” for Ixchiq use in those over 60 years of age, according to the notice.
Fox News Digital’s Melissa Rudy contributed to this report.
 Latest World Breaking News Online News Portal
Latest World Breaking News Online News Portal






